Malaysia Performs in Novo Nordisk Global Clinical Trials!
KUALA LUMPUR, Jan 2 — We are thrilled to shine the spotlight on some of the achievements noted in several of Novo Nordisk (NN) global studies conducted, that have Malaysian sites demonstrating outstanding clinical trial performance throughout 2024.
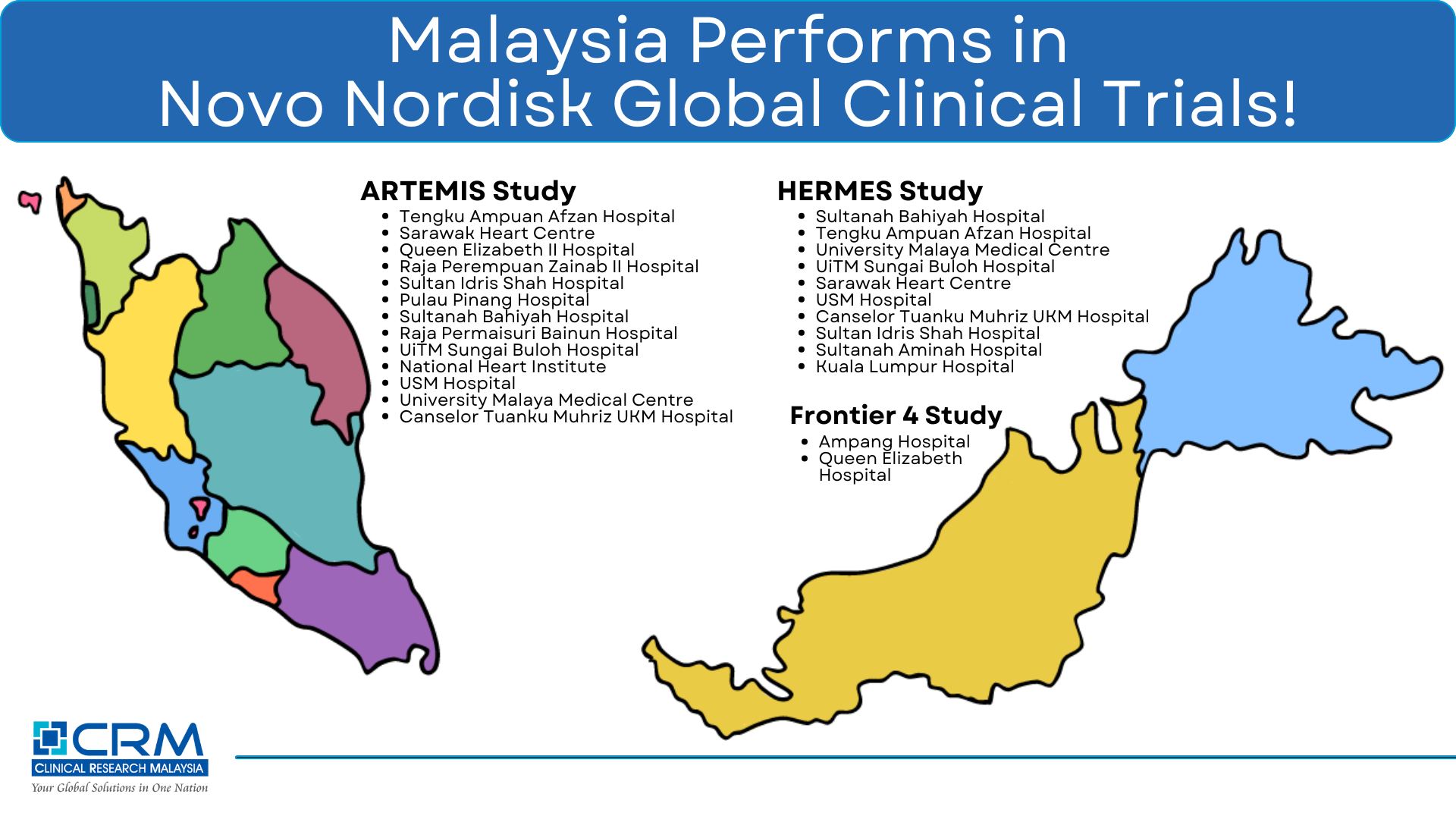
In the ARTEMIS study (a NN trial on Acute Myocardial Infarction), Malaysia is ranked among the top 3 countries globally, having been ahead in study activation, First-Patient-First-Visit (FPFV), and achieving its year-end enrollment target. In addition, Malaysia managed to exceed its year-end recruitment target by > 60%! Special mention to MOH hospitals participating in this global study (as listed below) for going above and beyond in ensuring the delivery of study with Speed, Quality & Reliability!
Hospital Tengku Ampuan Afzan, Kuantan: Top recruiting site in Malaysia and recruitment of trial participants within a short period. Shoutout to Investigator Dr Anwar Irawan and the Study Coordinators
Sarawak Heart Centre, Kuching: Instrumental role in experience sharing by the study team and great leadership by National Lead Investigator, Dr Alan Fong.
Queen Elizabeth II Hospital, Kota Kinabalu: 1st site activated globally and among the first 4 global sites to randomise trial participants. Also a top recruiting site in Malaysia. Shoutout to Investigator Dr Liew Houng Bang and National Study Coordinator Melanie
Hospital Raja Perempuan Zainab II (Kota Bahru), Hospital Sultan Idris Shah (Serdang), Hospital Pulau Pinang, Hospital Sultanah Bahiyah (Alor Setar) & Hospital Raja Permaisuri Bainun (Ipoh): All having the study activated at the site and achieving FPFV within 3 months.
In the HERMES study (a NN trial on Heart Failure), Malaysia overcame major challenges and exceeded recruitment expectations with 32 participants enrolled by year-end, making Malaysia the top recruiter in Southeast Asia. Notable contributions came from:
Hospital Sultanah Bahiyah (Alor Setar): Top recruiter in Malaysia. Led by Investigator Dr Abd Syukur Abdullah and supported by Study Coordinator Neoh Poh Tiew.
Hospital Tengku Ampuan Afzan, Kuantan: Exemplified resilience in overcoming recruitment challenges, due to trial complexity. In addition, the team is also involved in multiple NN Cardiovascular trials, striving in delivering good performance. Shoutut to Investigator Dr Anwar Irawan and Study Coordinators Murniyati & Zati Nabilah.
Final special mention is the Frontier 4 study (a NN trial on Hemophilia), in which Malaysia has successfully retained and rolled over all haemophilia patients from the previous Frontier 2, simultaneously meeting country targets and demonstrating excellence through participating sites like Hospital Ampang (Investigator Dr Jameela and Study Coordinator Wirdatul Ain) and Queen Elizabeth Hospital (Investigator Dr Lily Wong and Study Coordinator Tan Siew Phung).
All these achievements highlight the study teams’ exceptional teamwork, dedication, passion and leadership towards significantly contributing to global clinical research. Together, these trial sites have set new benchmarks in cardiovascular and haemophilia trials. To all the sites and teams, thank you for your outstanding contributions!
Comments are closed.